Refining of Metals
Refining of Metals: Overview
In this topic, we will learn how metals are refined. We will study various methods used for refining metals, such as distillation, liquation, poling, etc. It also describes the process of electrolytic refining here.
Important Questions on Refining of Metals
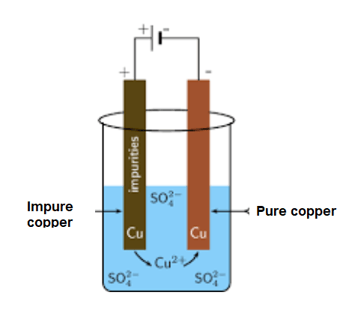
During the electrolytic refining of copper what happens at the anode?
Look at the image and answer the following question.
In the activity, a thin sheet of copper is made
Which of the following id used as reducing agent in the thermite reaction?
During electrolytic production of aluminium, the carbon anodes are replaced from time to time because
In Alumino-Thermite process aluminium is used as
The metal which can be refined by liquation is
Which of the following is not a method of purification of metals?
In thermite reaction, a metal oxide is reduced to metal using a metal '' as a reducing agent. The metal '' refers to
The chemical formula of magnetite is _____.
:
:
:
Enter your correct answer as or .
Write the cathodic reaction involved in the extraction of potassium from potassium chloride.
Write the anodic reaction involved in the extraction of potassium from potassium chloride.
The anode used in the electrolysis of potassium chloride is: _____
The cathode used in the electrolysis of potassium chloride is:
Describe the extraction of potassium from potassium chloride.
In the Down's process of extraction of sodium from molten sodium chloride, the reason for adding anhydride calcium chloride is:
The gas which gets collected at the anode in the electrolysis of molten is:
During the extraction of sodium from brine, water molecules undergo the following reaction:
On which electrode will this reaction take place?
